

Genmark is not profitable, though it is moving that way. Roche also makes a cobas Sars-Cov-2 & Influenza A/B nucleic acid test for use on the cobas 68 machines no problem has been identified with this product, or any other of Roche’s half-dozen or so Covid-19-related tests that have also been authorised by the FDA. The assay tubes might sporadically leak, potentially leading to invalid results or false positives, particularly for the Flu B test and abnormal PCR cycling in the reaction tubes, which could cause false positives for multiple analytes in a single testing run. The agency said Roche had identified two issues with the cobas Sars-Cov-2 & Influenza A/B nucleic acid test for use on the cobas Liat system. On Friday the FDA wrote to doctors to warn of potential false positives with Roche’s similar test. And it will be happy to come to the rescue, since its own multi-virus diagnostic is in a spot of bother. Indeed, demand for the panel is such that it is outstripping the company’s manufacturing capacity – an area in which Roche will surely be able to help.
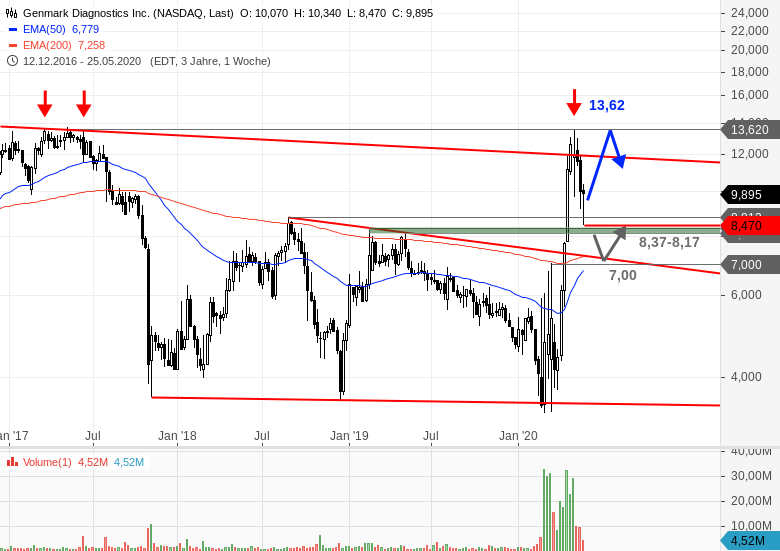
Demand for the RP2 panel itself drove “a large portion” of Genmark’s $172m 2020 revenue, according to Scott Mendel, the group’s chief executive.
Last year Genmark sold 265 ePlex systems – the machines on which the panel runs – increasing its installed base by 50%. Genmark’s ePlex respiratory pathogen panel 2, authorised by the FDA in October, is capable of detecting and differentiating nucleic acid from the novel coronavirus as well as nine kinds of flu or parainfluenza, the four coronaviruses that cause the common cold, and several other viruses and bacteria. The challenge for Roche will be maintaining a respectable rate of growth as the pandemic wanes. Those revenues had nearly doubled from 2019, thanks in part to Genmark’s Covid-19 panel. This morning the Swiss group bought Genmark Diagnostics for $1.8bn in cash, paying a 30% premium to Genmark’s closing share price on Friday and more than 10 times its 2020 revenues. The timing is coincidental, but it will be reassuring to Roche’s shareholders that the company has acquired a maker of a combined test for Covid-19 and other respiratory pathogens just as its own combo Covid-19 and flu test has hit a snag.


 0 kommentar(er)
0 kommentar(er)
